aetokthonotoxin (AETX) qPCR Detection Kit (real-time PCR kit for the AetA gene)
$376.25
In Stock & Ready to Ship
This kit is sufficient for 150 reactions:
- Real time qPCR kit for AetA gene
- For screening aetokthonotoxin gene cluster
- Use in combination with Attogene Algae DNA isolation kit
Compatible with automated systems.
Not all cyanobacterial strains produce toxins. However, the toxin-producing strains cannot be distinguished from the nontoxin-producing strains by traditional light microscopy, commonlyused to monitor water bodies. An alternative for the differentiation of potentially toxic strains from nontoxic strains is to use molecular methods to detect the presence of toxin biosynthetic genes. Such methods are already available and could be used for the detection and identification of potential microcystin and nodularin producers present in environmental samples (Attogene catalog number NA2024).
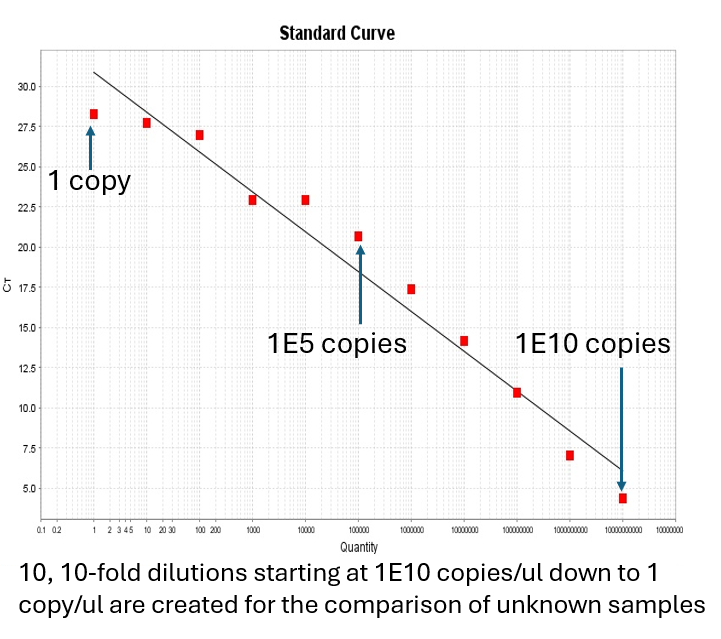
Screening for the toxin itself, can be very costly. In turn, real time PCR for the detection of a gene region responsible for assembling in cyanobacterial strains and environmental samples can be a key indicator for the prescense of cyanobacteria capable of expressing the aetokthonotoxin toxin. Attogen has thus, designed primer pairs and probes targeting a the conserved gene region in order to enable the amplification and detection of several producer genera using real time PCR. Screening for the toxin genes can save significant costs and act as a triage for samples needing to be analyzed for the toxin itself.
Cyanobacterial neurotoxin aetokthonotoxin (AETX), a peculiar pentabrominated biindole alkaloid implicated in fatal Vacuolar Myelinopathy. This neurodegenerative disease was first recorded in 1994 during an outbreak of bald-eagle poisonings at De Gray Lake in Arkansas, USA. AETX was experimentally confirmed to be produced by the true branching heterocytous cyanobacterium Aetokthonos hydrillicola. The production of AETX is dependent on bromide (Br−) availability, and likely linked to its hyper-accumulation by the host plan. Thus regular monitoring of A. hydrillicola (accompanied by assessment of Br− and AETX levels) is highly advisable to predict the possible threat of further VM outbreaks.
The cyanobacterial AetA gene which encodes the unique FAD-dependent halogenase involved in the pathway for AETX synthesis has been adapted to develop a -aetokthonotoxin specific quantitative PCR (qPCR) assay.
You may also like…
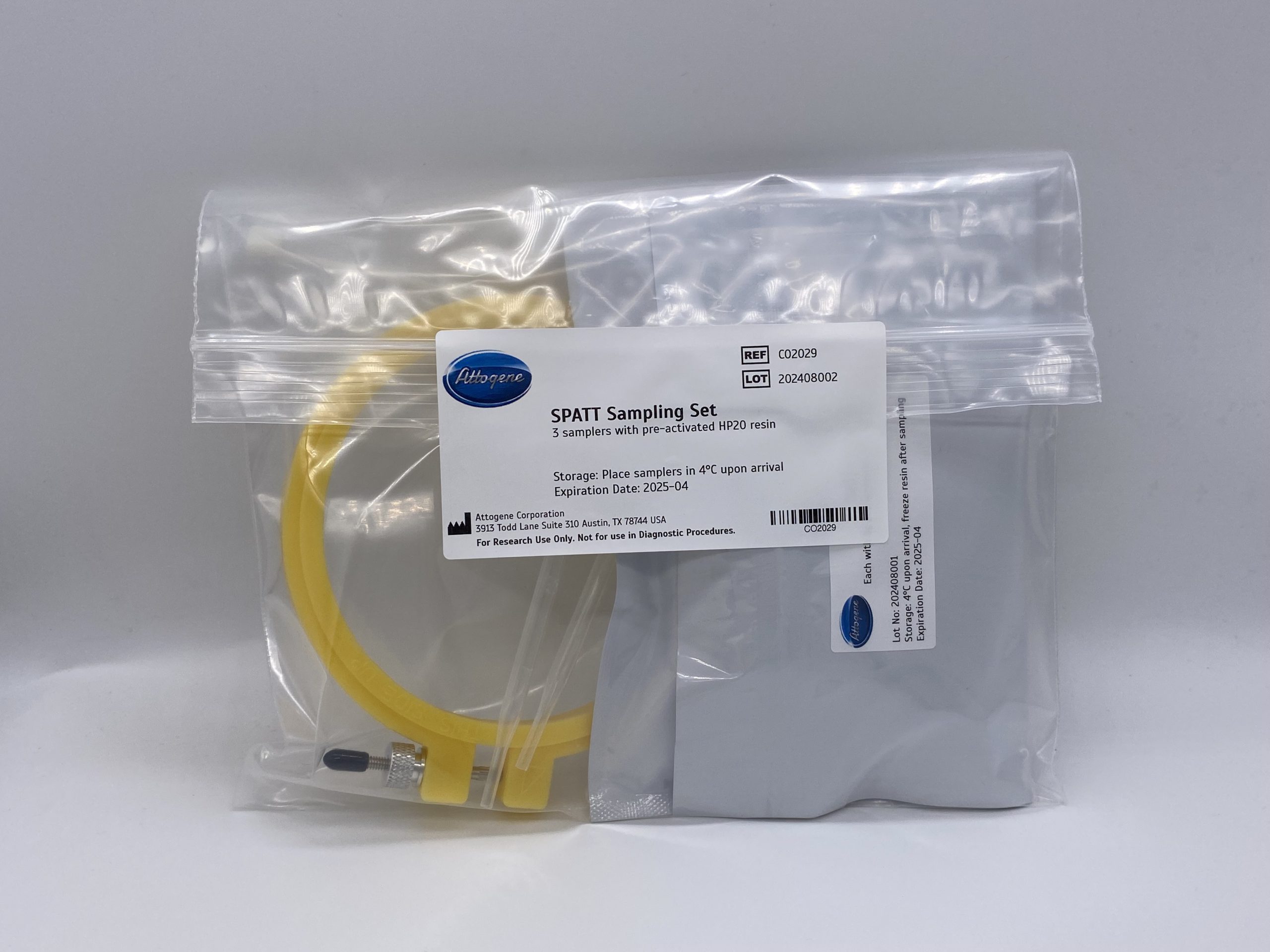
Solid Phase Adsorption Toxin Tracking (SPATT) Bag Set
$80.62- Set of three Solid Phase Adsorption Toxin Tracking (SPATT) Bags
- Pre activated
- Ready for deployment
- HP20
Microcystin qPCR Detection Kit (real-time PCR kit for MycE Cluster)
$376.25This kit is sufficient for 150 reactions:
- Real time qPCR kit
- For screening microcystin gene cluster
- Use in combination with Attogene Algae DNA isolation kit
Compatible with automated systems.
Plant and Algae RNA Isolation Kit
$455.80This kit is sufficient for 100 RNA isolations based on:
- 200mg fresh plant
- 1ml algae culture
- 50mg dry seeds.
Compatible with automated systems.