About Attogene
Attogene is a rapidly evolving biomanufacturing company located in Austin, Texas USA. Our focus is to develop and make customer focused products to improve global health and the environment.
What We Do
- Develops new and novel cutting-edge technologies for sensing compounds in diverse sample types.
- Develops and sell assays for the detection of environmental compounds and human disease analytes.
- Offer diverse services which help our customers with unique assay and application needs.
- Offer kits from hand selected partners: Primer Design (one of the first EUA FDA authorized COVID PCR diagnostic test kit), and GenomeMe
“I highly recommend Attogene to anyone looking for various types of test strips, ELISAs, PCR, nucleic acid and other types of biological and chemical assays.”
– John G. Bruno, Ph.D., Director of Biotechnology, Nanohmics Inc.Austin, TX
“Attogene helped our company commercialize new technology for our sector. We are planning a phase two of this project in the upcoming months. We recommend using Attogene.”
-Jeff P.Houston, TX
Featured Products
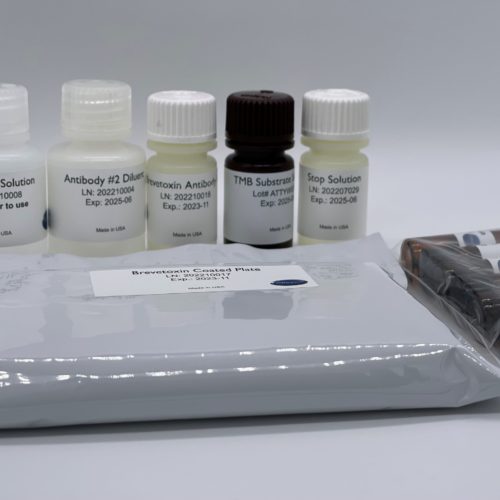
Brevetoxin ELISA Kit (Neurotoxic Shellfish Poisoning)
$425.00- Format: 96-well microtiter plate (12 test strips of 8 wells)
- Standards: 0 | 0.1 | 0.25 | 0.5 |1.0 | 2.6 ppb
- Incubation Time: 75 Minutes
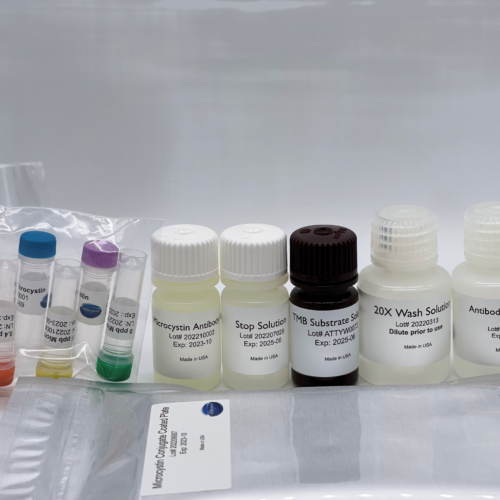
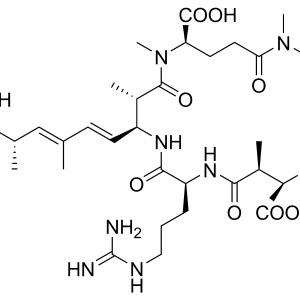
Congener-Independent Microcystin ELISA Kit
$480.00- Format: 96-well microtiter plate (12 test strips of 8 wells)
- Standards: 0 | 0.05 | 0.1 | 0.2 | 0.4 | 2 ppb
- Incubation Time: 75 Minutes
Microcystin ELISA Kit
$415.00- Format: 96-well microtiter plate (12 test strips of 8 wells)
- Standards: 0 | 0.05 | 0.1 | 0.2 | 0.4 | 2 ppb
- Incubation Time: 75 Minutes
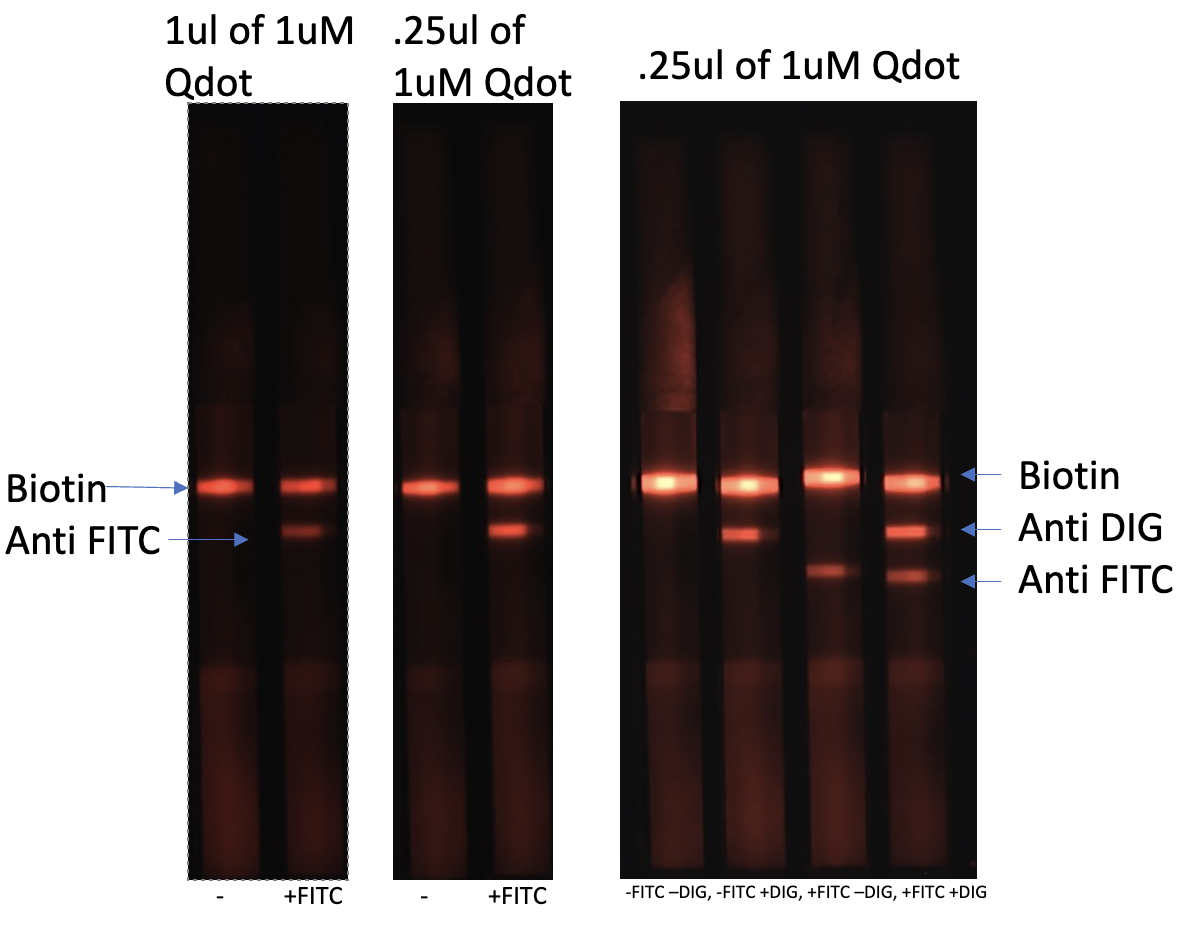
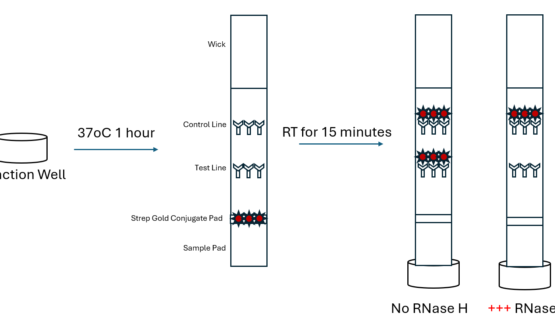
Streptavidin CdSe/ZnS Quantum Dots For Lateral Flow
$350.00Highly stable and uniform Streptavidin CdSe/ZnS Qdots. The quality and performance of a conjugate is critical to successful lateral flow test manufacturing. Our products are produced in a state-of-the-art manufacturing facility that enable rapid turnaround times while ensuring batch to batch consistency and reliability.