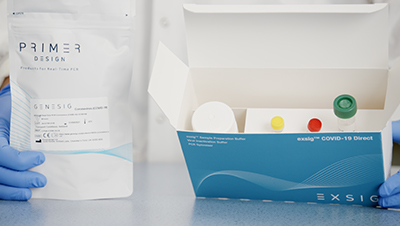
exsig® COVID-19 Direct
$2,103.08
In Stock & Ready to Ship
A total workflow solution with all reagents and components provided from start to finish.
- Total workflow solution
- All reagents and components provided from start to finish
- Rapid protocol with fewer handling steps
- For use with dry swabs
- Viral inactivation; Preparation, Amplification, Diagnosis
- Total chemistry optimisation
- Low shipping costs with ambient shipping
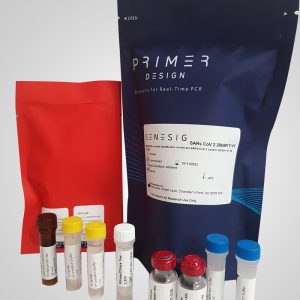
- Includes our genesig® Real-Time PCR Coronavirus COVID-19 (CE IVD)
The genesig® Real-Time PCR COVID-19 (CE) is CE-IVD marked and intended for in vitro diagnostic use in Europe.
PLEASE NOTE: This is a Professional Use Only product that requires a trained operator in a controlled laboratory environment. It is NOT a lateral flow or a Point of Care device designed to be used by the general public.