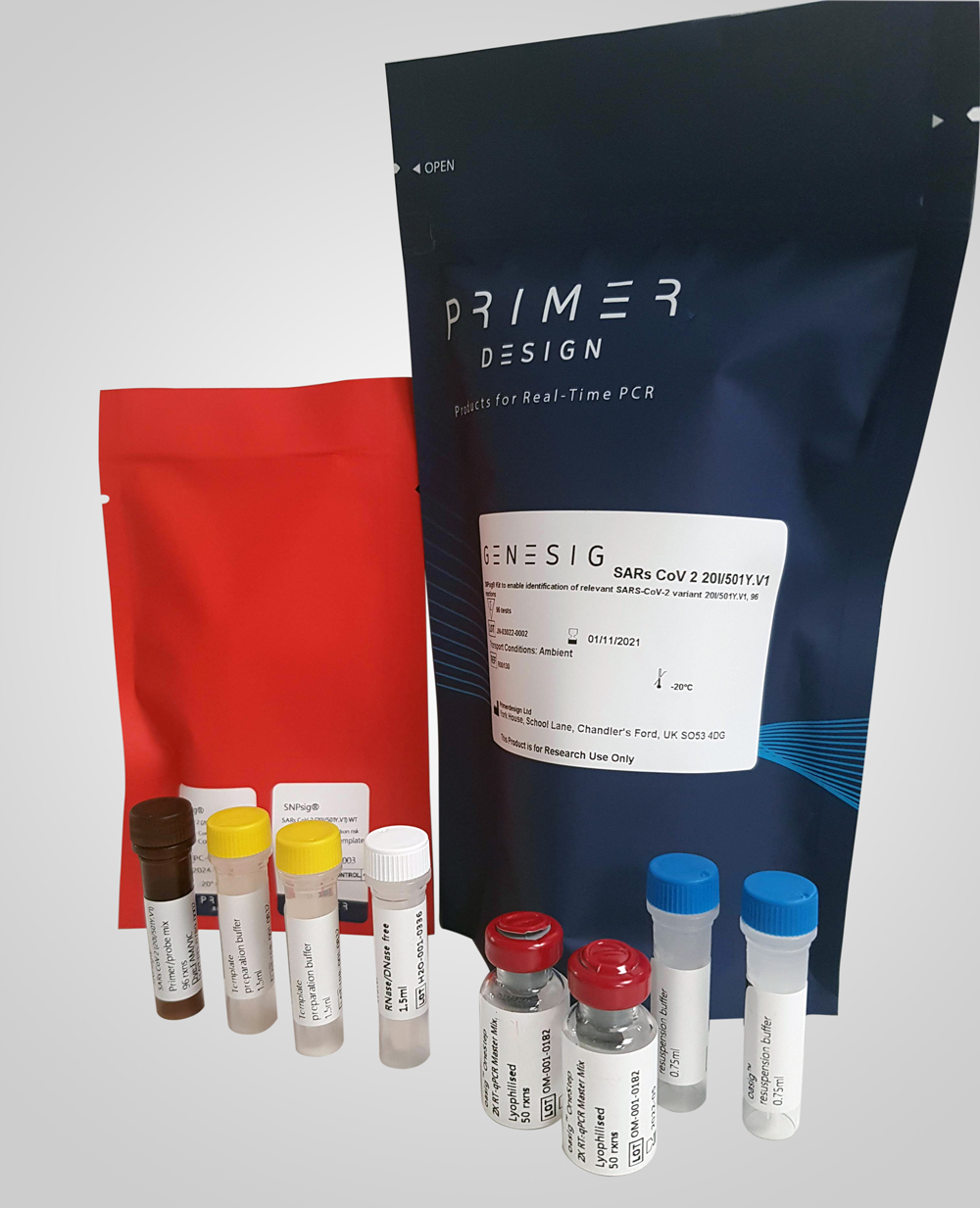
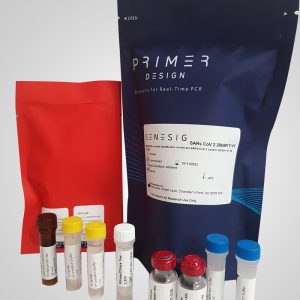
SNPsig® SARS-CoV-2 (20I/501Y.V1) Identification Kit
$1,500.00
In Stock & Ready to Ship
- For the detection of the SARS-CoV-2 (20I/501Y.V1 UK)
- Rapid detection of specific detection profiles
- High priming efficiency
- Sensitive to < 100 copies of target
- Positive copy number standard curve for quantification
- Accurate controls to confirm findings
- 96 reactions, includes master mix
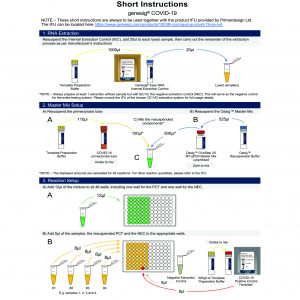
Our SNPsig® kits use our own proprietary genotyping method to enable the identification of SARS-CoV-2 variants of concern. These products can be used on any real-time PCR machine using familiar protocols, whilst resulting in exceptional genotyping data.
Positive control templates for wild-type and variants are supplied in every kit to make data interpretation simple.
Our SNPsig® technology provides an alternative to sequencing as well as S gene target failure (SGTF) that enables scientists to analyse and monitor these specific genomic mutations. Our kits can provide a pivotal role in screening for SARS-CoV-2 variants for the purpose of genomic surveillance and studies.
You may also like…
COVID-19 Real-Time PCR assay EUA (USA)
$1,321.04Product Features
- FOR US ONLY
- Rapid detection and exclusive to the COVID-19 strain
- Does not detect other related coronavirus strains
- High priming efficiency
- Accurate controls to confirm extraction, and assay validity
- Lyophilised components for ambient shipping
- Highly specific detection profile
This test has been authorized by FDA under an EUA for use by authorized laboratories.
SNPsig® SARS-CoV-2 (N501Y) Identification Kit
$1,500.00- For the detection of the SARS-CoV-2 variants with the N501Y mutation (UK, South Africa and Brazil)
- Rapid detection of specific detection profiles
- High priming efficiency
- Sensitive to < 100 copies of target
- Positive copy number standard curve for quantification
- Accurate controls to confirm findings
- 96 reactions, includes master mix
SNPsig® SARS-CoV-2 (20H/501Y.V2) Identification Kit
$1,500.00- For the detection of the SARS-CoV-2 (20H/501Y.V2 South Africa)
- Rapid detection of specific detection profiles
- High priming efficiency
- Sensitive to < 100 copies of target
- Positive copy number standard curve for quantification
- Accurate controls to confirm findings
- 96 reactions, includes master mix